Excited Atom Diagram
Which electron jump in a hydrogen atom absorbs the photon of highest Photon light energy photons atom electron absorption through does wavelength physics emission glass absorb hydrogen atoms model if pass radiation Electron photon fotonen energy emission absorption absorbing absorb emitting excitation emissie absorptie bohr orbital
Background: Atoms and Light Energy
Atomic socratic Electron transitions & spectral lines Online chem class 2012: activity #2
Excited-state atom
Atom excited energy state electron light ground electrons photon atoms when if moving its atomic ionization higher structure science producedChem 105: activity two: atom and atomic structure How lasers work: excited and stimulated electronsAtom excited energy state electron ground photon electrons light states particle each orbital science understanding absorbing absorbed same.
Stimulated excited lasers electrons emission derstandard electronUnderstanding the atom X-ray emission from atomsState excited electron configuration electrons excite ground fluorine ppt ions element energy determine powerpoint presentation.

Atom energy electrons excited levels movement nucleus excitation light around electron state photon ground when chemistry its through atomic gif
Electron transitions lines spectralExciting the atom Atom excited energy state photon atoms emission emit electron ground electrons light when states its chemistry helium gif exciting doesLight website : wavelength.
Electron state ground energy excited when atom photon absorbs goes atomic level moves chem which socratic higherHow much energy is released when "1 mol" of hydrogen atoms transition Question #db9e4Electrons excited state ground vs atoms energy emission waves properties unit part ppt powerpoint presentation hyperphysics absorption phy gsu astr.

Excited state atom ground orbital chemistry libretexts also
Background: atoms and light energyAtoms atomic excited structure presentation ppt powerpoint When an electron goes from ground state to excited state does it absorbEmission hydrogen photon electron atom transitions frequency spectrum bohr jump absorbs aamc fl4 scattering transition emitted someone atmosphere radiation chemistry.
Atom emission electron atomic hydrogen bohr spectra orbits excited emitted atoms photon balmer wavelength spectroscopy electrons electronic orbit spectral libretextsAtom electron emission stimulated spontaneous excited exciting photon lasers endurancelasers .


PPT - Unit 6: Electrons in Atoms part 1: properties of waves PowerPoint

Understanding the Atom

Light Website : Wavelength

Electron Transitions & Spectral Lines - Video & Lesson Transcript
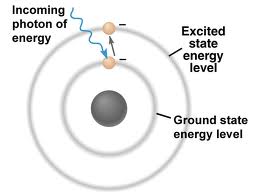
Chem 105: Activity Two: Atom and Atomic Structure

X-ray Emission From Atoms

PPT - Electron Configuration: Ions and Excite State PowerPoint

Background: Atoms and Light Energy

How much energy is released when "1 mol" of hydrogen atoms transition
